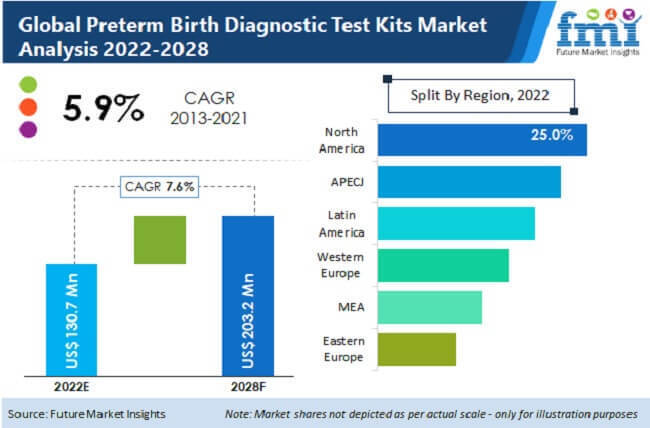
[257 Pages Report] According to Future Market Insights (FMI), the global Preterm Birth Diagnostic Test Kits Market was worth US$ 121.3 Mn in 2021 and is expected to grow 1.6X during the forecast period. During the forecast period of 2022-2028, the market is expected to grow at a CAGR of 7.6%, with sales of US $ 203.2 Mn in 2028.
A new study by the company titled ‘Preterm Birth Diagnostic Test Kits Market: Global Industry Analysis 2013-2017 and Opportunity Assessment 2018-2028’, has listed out the key points being considered by preterm birth diagnostic test kits companies to emerge and stay sustainably profitable in the long run in the preterm birth diagnostic test kits market.
The global preterm birth diagnostic test kits market is expected to expand at a CAGR of 7.4% over the forecast period to reach a value of over US$ 200 Mn by 2028, and the North America preterm birth diagnostic test kits market is expected to remain as a main sink market for revenues. Europe is expected to remain as the main source market for preterm birth diagnostic test kits, as most of the preterm birth diagnostic test kits facilities are located in this region. However, with the increasing demand and cost-effective diagnostic options in countries such as China and India, and various countries of Asia Pacific and Middle-East, is fueling the growth of preterm birth diagnostic test kits market.
A sample of this report is available upon request @ https://www.futuremarketinsights.com/reports/sample/rep-gb-7551
Key Findings Presented in the Report on the Preterm Birth Diagnostic Test Kits Market
The report finds that approximately 115.4 million births occurred in 2017, out of which 11.1% births were preterm, which is approximately 12.8 million births. As per WHO, preterm birth complications are the leading cause of deaths amongst children under the age of 5, directly responsible for approximately 1 million death in 2015. Out of these, 3/4th of these deaths can be prevented with cost-effective interventions. The adoption rates for preterm birth diagnostic tests kits ranged from 2% to 15% distributed over various geographies. Majority of the existent patient pool are unaware about the cost-saving benefits of preterm birth diagnostic test kits, thus, a major portion on the preterm birth test kits market has remained untapped which remains open for manufactures of preterm diagnostic test kits to exploit the preterm birth diagnostic test kits market over the forecast period.
Analysis of the Preterm Birth Diagnostic Test Kits Market
For preterm birth diagnostic test kits, blood sample is the most common sample take for diagnosing preterm labor and premature rupture of membrane. Blood sample-based preterm birth diagnostic tests in preterm cases are the most common and widely accepted in the preterm birth diagnostic test kits end users. However, vaginal discharge sample type preterm birth diagnostic tests are gaining popularity for preterm birth diagnostic test kits.
Hospitals, outpatient clinics & diagnostic laboratories using preterm birth diagnostic tests are the primary point of contact for patients experiencing preterm birth symptoms. Hospitals are estimated to account for majority of the revenue share under the end users segment for preterm birth diagnostic test kits market in 2017, owing to being the primary healthcare facility for preterm cases. The prevalent cases of preterm birth, growing demand for cost-effective diagnostic options and preterm birth test kits, and success in clinical studies for developing a rapid preterm birth diagnostic test kits is expected to fuel the growth of the global preterm birth diagnostic test kits market over the forecast period.
Ask An Analyst @ https://www.futuremarketinsights.com/ask-the-analyst/rep-gb-7551
Activities across the preterm birth diagnostic test kits manufacturers are restricted to efficiency and specificity testing. The top activity associated with preterm birth diagnostic test kits manufacturers is joint collaboration with various organizations in order to spread awareness, further development, and test the sensitivity, specificity and efficiency of preterm birth diagnostic test kits.
Factors Impacting Growth of the Preterm Birth Diagnostic Test Kits Market
Rising focus on cost-effective diagnosis using preterm birth diagnostic test kits is expected to boost the growth of the preterm birth diagnostic test kits market. Preterm birth diagnostic test kits manufacturing companies focusing on spreading awareness about preterm birth diagnostic test kits and marketing preterm birth diagnostic test kits through campaigns and joint research studies is expected fuel the growth of the preterm birth diagnostic test kits market during the forecast period. However, Preterm birth diagnostic kits experience stiff competition from alternative product lines. Trans-vaginal scans, advanced ultrasound are some of the examinations adopted by healthcare providers over preterm birth diagnostic kits and would pose challenges to the growth of the preterm birth diagnostic test kits market.
Preterm Birth Diagnostic Test Kits Market: Companies
Some of the key preterm birth diagnostic test kits companies analyzed in this report titled Preterm birth diagnostic test kits market include Creative Diagnostics, Qiagen, Medixbiochemcia, Hologic Inc, Sera Prognostics, IQ Products, Biosynex, Nanjing Liming Biological Preparations Co Ltd, Clinical Innovations LLC, BIOSERV Diagnostics GmbH, Wuxi BioHermes Biomedical Technology Co. Ltd, and Anhui Deep Blue Medical Technology.
For in-depth competitive analysis, Buy Now – https://www.futuremarketinsights.com/checkout/7551
Preterm Birth Diagnostic Test Kits Market, By Category
Product:
- ffN Test
- PAMG-1 Test
- IGFBP-1 Test
Sample:
- Blood
- Urine
- Vaginal Discharge
End User:
- Hospitals
- Diagnostic Laboratories
- Outpatient Clinics
- Research Centers
More Trending Reports by Future Market Research :
Aflatoxicosis Treatment Market Forecast : As per the analysis, the global aflatoxicosis treatment market is expected to secure US$ 403.5 Million from 2022 to 2032, while recording a CAGR of 3.8% during the prior mentioned time period.
Pancreatic Stone Protein Testing Market Value : The pancreatic stone protein testing market is likely to record a strong CAGR of 1.8% during the forecast period. The pancreatic stone protein testing market is currently valued at US$ 3 Bn in 2022 and is likely to reach US$ 3.59 Bn by 2032.
Multiple Unit Pellet Systems Market Sales : The global multiple unit pellet systems (MUPS) market was valued at US$ 3.48 Bn in the year 2021, expanding at a CAGR of 3.5% to reach an ~US$ 5.07 Bn by the end of 2032.
About FMI:
Future Market Insights (ESOMAR certified market research organization and a member of Greater New York Chamber of Commerce) provides in-depth insights into governing factors elevating the demand in the market. It discloses opportunities that will favor the market growth in various segments on the basis of Source, Application, Sales Channel and End Use over the next 10-years.
Contact Us:
Future Market Insights,
Unit No: 1602-006,
Jumeirah Bay 2,
Plot No: JLT-PH2-X2A,
Jumeirah Lakes Towers,
Dubai,
United Arab Emirates
For Sales Enquiries: [email protected]
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs
The post Preterm Birth Diagnostic Test Kits Market is anticipated to expand at a CAGR of 7.6% from 2022-2028 and will reach U.S. $ 203.2 Mn by 2028 appeared first on Future Market Insights.